The bodies of all organisms are composed of variety of chemical compounds. These chemical compounds are formed by the bonding of naturally existing elements in different ways.
There are only about 25 elements in the living body out of the 92 elements present in nature. They are present at different locations in the body in different forms.
The most common 4 elements in the living body are Carbon, Hydrogen, Oxygen and Nitrogen. Other than above Sulphur, Phosphorous, Sodium, Potassium, Calcium, Magnesium, Iron and Chlorine are essential for the survival of organisms.
Figure 1.1 shows the percentages of main elements in the human body.

Chemical compounds that build up living matter can be divided into two categories as organic compounds and inorganic compounds. Compounds which contain Carbon are known as organic compounds and compounds which do not contain Carbon are known as inorganic compounds. (Carbon dioxide, Carbon monoxide, Carbonates and Bicarbonates are some of the inorganic compounds which contain Carbon) Those organic compounds that build up the living body or living matter are known as bio molecules. They are:
- Carbohydrates
- Proteins
- Lipids
- Nucleic acids
These are considered as the main types of bio molecules. Instead of these four types, Vitamins are also one of the organic compounds found in living matter.
Water, minerals and gases are some of the inorganic molecules that are essential for the maintenance of life.
Carbohydrates
This is the most abundant organic compound on earth. They are produced during the photosynthesis of green plants. Potato, sweet potato, grains, sugar, flour are
some of the examples for foods which contain carbohydrates.
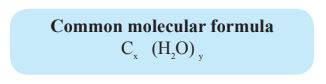
The main elemental composition of carbohydrates is Carbon (C), Hydrogen (H) and Oxygen (O).
Hydrogen and oxygen combine in 2:1 ratio in carbohydrates.

Disaccharides
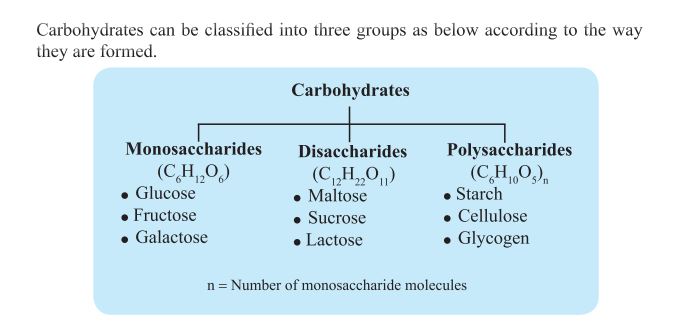
Two Monosaccharides join to form a Disaccharide. During this process a water molecule is released. In the same way relevant Monosaccharides can be obtained
by hydrolyzing Disaccharides. Disaccharides are sweet, water soluble crystals.

Maltose, Sucrose and Lactose are examples for disaccharides. The characters of disaccharides are discussed in the table below.

Polysaccharides
Polymerisation of a large number of monosaccharides form a polysaccharide molecule. Hydrolysis of Polysaccharide results relevant monosaccharides. Insoluble in normal water. They are not crystals. Cellulose, Starch and Glycogen are examples for polysaccharides. The structural unit of Cellulose, Starch and Glycogen is Glucose , but their properties are different according to the number of Glucose molecules and how they are bound with each other.
The characters of polysaccharides are discussed in the table below.

Significance of Carbohydrates
- As an energy Source – The main source to obtain energy for the activities of or ganisms is t he c arbohydrate.The Monosaccharides (Glucose) produced due to hydrolysis of those compounds release energy.
- As a storage compound
- As a structural component in plant cell wall
- As a constituent of Nucleic acid